
EMA Marketing Authorization of New Drugs in February 2025
Shots:
-
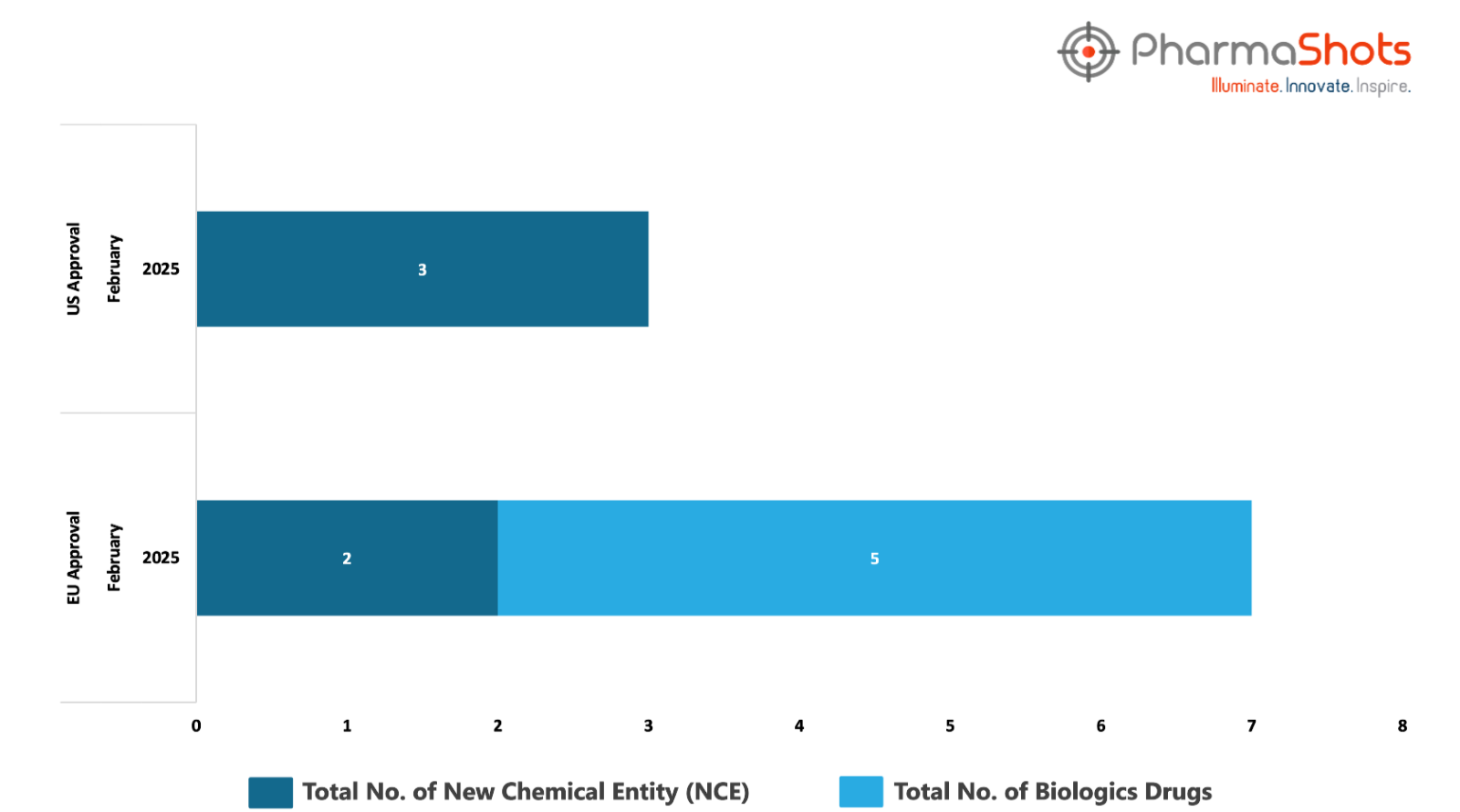
The EMA’s CHMP has granted positive opinions and approvals to 5 Biologics and 2 New Chemical Entities in February 2025, leading to treatments for patients and advances in the healthcare industry
-
The major highlighted drugs were Merck’s Welireg to treat Von Hippel-Lindau & Renal Cell Carcinoma
-
PharmaShots has compiled a list of 6 drugs that have been granted positive opinions and approvals by the EMA’s CHMP & EC, respectively
Company: Galderma
Product: Nemluvio
Active Ingredient: Nemolizumab
Disease: Prurigo Nodularis and Atopic Dermatitis
Date: Feb 12, 2025
Shots:
-
Based on ARCADIA and OLYMPIA studies EU approved Nemluvio for moderate-to-severe AD and PN and is now approved for SC use in pts. aged 12 and older for AD and in adults for PN who are candidates for systemic therapy
-
ARCADIA 1 & 2 studies assessed Nemluvio (Q4W) + TCS ± TCI vs PBO in atopic dermatitis patients (n=1,728) for 16 & 48wks., respectively. It showed improved co-1EPs & all key 2EPs as well as itch relief at wk.1
-
OLYMPIA 1 & 2 trials assessed Nemluvio (Q4W) vs PBO in PN patients (n=560) for 16 & 24wks., respectively. It achieved its co-1EPs & all key 2EPs, depicting improved itch & skin nodules at wk.16, with fast itch reductions at wk.4
2. CSL and Arcturus Therapeutics Report the EC’s Approval of Kostaive Against COVID-19
Company: CSL and Arcturus Therapeutics
Product: Kostaive
Active Ingredient: Zepomeran
Disease: COVID-19
Date: Feb 14, 2025
Shots:
-
The EC has approved Kostaive (ARCT-154) for active immunization to prevent COVID-19 in subjects of age ≥18yrs in the EU & EEA states following CHMP positive opinion in Dec 2024
-
Approval was based on Kostaive's clinical data incl. P-I/II/III showing its efficacy & tolerability, plus P-III COVID-19 booster trial depicting superior immunogenicity vs mRNA comparator. Also, follow-up analysis of booster dose showed higher immunogenicity & antibody response for ~12mos. in adults
-
Kostaive is a self-amplifying mRNA vaccine that codes for the SARS-CoV-2 spike protein to provide active immunization against COVID-19
Company: Merck
Product: Welireg
Active Ingredient: Beremagene Geperpavec
Disease: Von Hippel-Lindau & Renal Cell Carcinoma
Date: Feb 18, 2025
Shots:
-
The EC has approved Welireg for VHL-related localized RCC, CNS hemangioblastomas, or pNET unsuitable for localized procedures and advanced ccRCC post-PD-1/PD-L1 inhibitors or VEGF therapies progression across EU member states, as well as Iceland, Liechtenstein & Norway.
-
Approval for VHL-related tumors was based on P-II (LITESPARK-004) study (n=61), showing ORR of 49% (all PRs), with 56% maintaining response (RCC); 63% (CR: 4%, PR: 58%), with 73% maintaining response (CNS hemangioblastomas) & 83% (CR: 17%, PR: 67%), with 50% maintaining response for ≥12mos. (pNET); mDoR not reached.
-
Approval for ccRCC was based on P-III (LITESPARK-005) study (n=746), depicting reduced disease progression or death risk by 25% vs everolimus, with mPFS of 5.6mos., ORR of 22% (CR: 3%, PR: 19%) vs 4% (PR: 4%)
4. Gilead Receives EC’s Conditional Marketing for Seladelpar to Treat Primary Biliary Cholangitis (PBC)
Company: Gilead
Product: Seladelpar
Active Ingredient: Seladelpar
Disease: Primary Biliary Cholangitis
Date: Feb 20, 2025
Shots:
-
Following the MHRA approval (Jan 2025), the EC has granted conditional MAA to Seladelpar +/- ursodeoxycholic acid (UDCA) to treat PBC in those with inadequate response or intolerants to UDCA, respectively; ongoing regulatory review in Canada and Australia
-
MAA was based on a worldwide P-III (RESPONSE) study assessing seladelpar (10mg, QD, oral) vs PBO in PBC pts (n=193). It showed composite biochemical response in 62% vs 20% & ALP normalization of 25% at 12mos. (1EP) plus pruritus reduction of 3.2 vs 1.7 points at 6mos. (2EP)
-
Seladelpar (oral) is a PPAR- δ agonist that blocks bile acid synthesis to treat PBC
Company: Krystal Biotech
Product: Vyjuvek
Active Ingredient: Beremagene Geperpavec
Disease: Dystrophic Epidermolysis Bullosa (DEB)
Date: Feb 27, 2025
Shots:
-
The CHMP has recommended Vyjuvek (B-VEC) for treating wounds in DEB pts with COL7A1 mutations from birth. The EC's decision is expected in Q2’25, with launch anticipated under the trade name Vyjuvek in 30 EEA states—Germany in mid-2025 & France in late 2025
-
Opinion was based on various clinical data, incl. P-I/II (GEM-1) & P-III (GEM-3) trial data published in Nature Medicine & The NEJM, respectively, showing successful COL7A1 gene delivery & durable wound closure after topical application
-
Long-term safety & efficacy were supported by the US OLE trial & real-world data since Vyjuvek's US launch in 2023
Company: Regeneron
Product: Lynozyfic
Active Ingredient: Linvoseltamab
Disease: R/R Multiple Myeloma (MM)
Date: Feb 27, 2025
Shots:
-
The CHMP has recommended conditional approval of linvoseltamab for r/r MM pts who had ≥3 prior therapies (incl. proteasome inhibitor, immunomodulator, & anti-CD38 mAb) & had disease progression on last therapy, following the US FDA’s BLA acceptance (PDUFA: Jul 10, 2025)
-
Opinion was based on P-I/II (LINKER-MM1) trial data assessing linvoseltamab (200mg; QW then Q2W at 16wks. followed by Q4W if pts achieved VGPR or better at ≥24wks.) in 282 pts. Ongoing P-II dose expansion portion is evaluating ORR (1EP), with 2EPs of DoR, PFS, OS & MRD negativite rate
-
Also, Linvoseltamab is being studied in P-III (LINKER-MM3) trial as a monotx. & P-Ib (LINKER-MM2) trial to treat r/r MM in combination with other cancer treatments
Note: The following drugs have also been recommended for approval, however, no PR was available:
-
Deqsiga (human normal immunoglobulin)
Related Post: Insights+: EMA Marketing Authorization of New Drugs in January 2025
Tags

A passionate content writer with expertise in delivering high-quality and engaging content, Dipanshu is a keen reader and a versatile writer. Dipanshu dedicatedly covers news ranging from biopharma, life sciences, biotech, and MedTech to diagnostics and animal health companies, FDA, EMA, and biosimilar approvals. He can be contacted at connect@pharmashots.com














